To read more about the ongoing rights issue and register your interest, please press the button below.
Prolight Diagnostics, together with our subsidiary Psyros Diagnostics Ltd., develops innovative Point-of-Care (POC) systems. These are small, portable instruments and disposable cartridges for performing in-vitro diagnostic (IVD) tests from a drop of blood.
POC tests are performed outside the traditional hospital laboratory, for example in an emergency department, ambulance, healthcare centre or nursing home. POC tests are generally easier to perform than central lab tests and do not require complex training.
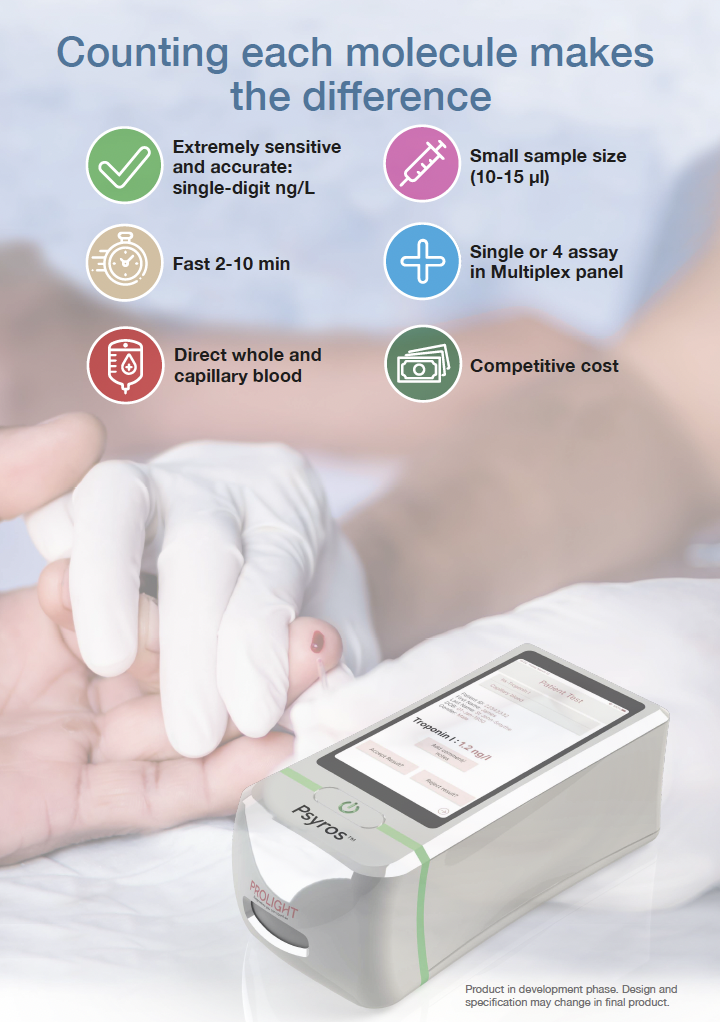
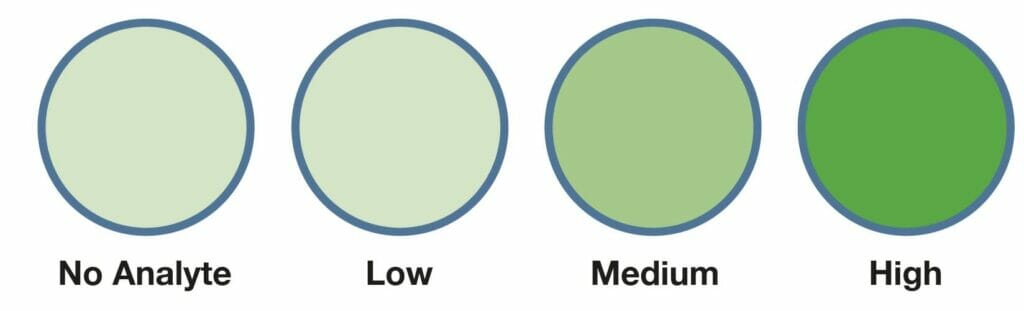
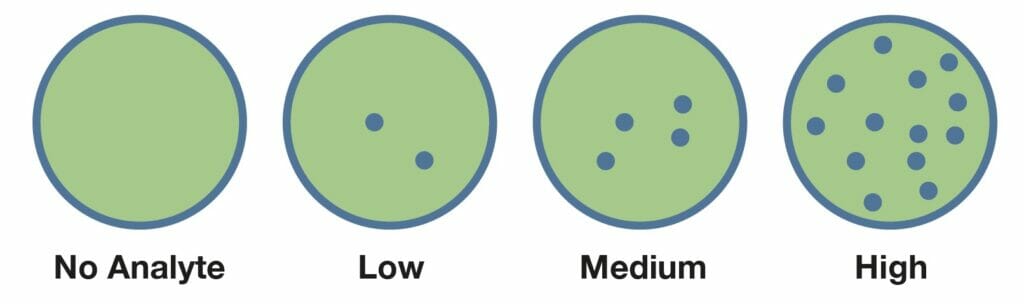
The novel Psyros™ digital (single molecule counting) technology provides the opportunity to develop new ultra-sensitive POC tests in many clinical areas previously limited to specialized laboratories.
Potential future applications in large clinical areas:
Product in development phase. Design and specification may change in the final product.
